Reports
KX2-391 Ointment 1%, a Novel Dual Sarcome/Tubulin Inhibitor, is Efficacious and Safe in the Treatment of Adults with Actinic Keratosis in Two Phase 3 Studies
Presented by: Edward Lain, MD, FAADAustin Institute for Clinical Research Inc., Pflugerville, TX, USA
- KX2-391 Ointment 1% is safe and effective in the treatment of adults with actinic keratosis (AK)
- KX2-391 Ointment 1% demonstrated 100% AK clearance in at least 44% of patients studied.
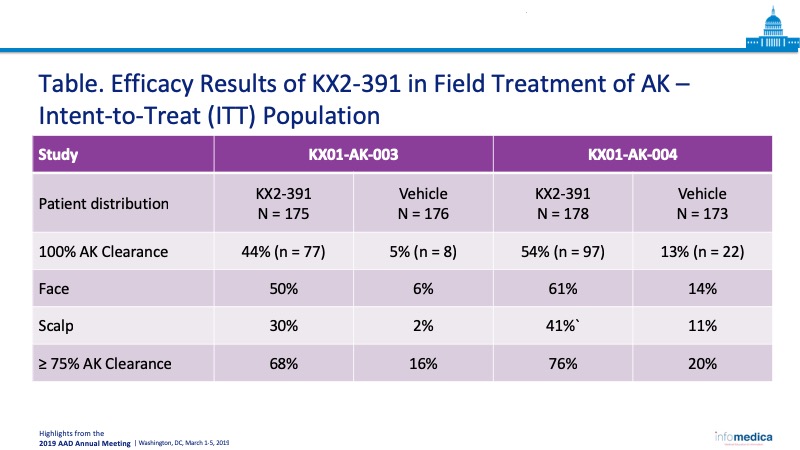
KX2-391 Ointment 1%, a novel dual sarcome (Src)/tubulin inhibitor, was shown to be efficacious in the treatment of adults with actinic keratosis (AK) in two phase 3 studies (KX01-AK-003 and KX01-AK-004). Primary outcome measures for the study consisted of 100% AK clearance, or partial clearance specific to the face or scalp. KX2-391 Ointment 1% demonstrated statistically significant clinical benefit over placebo, with 44% of KX01-AK-003 patients and 54% of KX01-AK-004 patients reporting 100% AK clearance. Outcome measures for safety metrics demonstrated that treatment-related emergent adverse events (TEAEs) were mild-to-moderate.
To assess the primary efficacy endpoint of 100% clearance of AK at day 57, and to assess the safety, local skin reactions (LSRs) and adverse events (AEs) of KX2-391 ointment 1% versus vehicle in adults with AK.
Type of Study
- Double-blind.
- Vehicle-controlled.
- Randomized.
- Parallel group.
- Multicenter.
- Phase 3.
Primary Purpose
- Assessment of primary efficacy endpoint of 100% clearance of AK at day 57 for KX2-391 ointment 1% versus vehicle in adults with AK.
- Assessment of the safety (local skin reaction and (AEs) of KX2-391 ointment 1% versus vehicle in adults with AK.
Patient Populations
- Total number of enrollees: 702 subjects in two identical studies, comprising 351 patients each located at 31 sites per study.
- Intent-to-treat population.
- Median Age: 69.75 years.
- Sex: Male/Female ratio of 6.7 to 1.
- Race: 99.5% white.
- Fitzpatrick skin type I/II composition: 72.75%.
- Median baseline AK lesion count: 6.
- Treatment area: Face to Scalp ratio: 2:1
Primary Outcome Measures
- The primary outcome measure was 100% clearance of AK at day 57 within the 25cm2 area in adult AK patients using KX2-391 ointment 1% versus vehicle.
Secondary Outcome Measures
- Assessment of safety data specific to local skin reaction and AEs of KX2-391 ointment 1% versus vehicle in adults with AK.
Drugs/Procedures Used
- KX2-391 is a small molecule with differentiated mechanisms of action, including inhibition of Src kinase activity and inhibition of tubulin polymerization that leads to an upregulation of apoptosis in proliferating cells.
- The treatment area was 25 cm2 with 4-8 typical visible AK lesions on face or scalp.
- KX2-391 ointment 1% vs. vehicle at 1:1 ratio.
- Treatment occurred once daily for 5 consecutive days, applied by study participants and left in place for approximately 12 hours.
- Treatment compliance greater than 99% was achieved.
- Outcome measures for efficacy demonstrated a statistically significant effect from KX2-391 treatment versus vehicle (Table).
- Outcome measures for safety demonstrated that KX2-391 was well tolerated.
- No ocular exposure led to ocular adverse events.
- There were no related clinically significant abnormal EKGs, changes in physical exams, or, vital signs, or laboratory findings.
- All serious adverse events (SAEs) that occurred during the study were considered unrelated to the treatment.
- The proportion of TEAEs were 11-20% in the active group versus 9-11% in the vehicle only group.
- The mean composite LSR sum of 4 occurred at day 8 and resolved on average by day 29, with the most common LSR occurrences consisting of erythema, flaking or scaling.
- KX2-391 ointment 1% was shown safe and effective in two identical phase 3 trials, demonstrating higher overall complete AK clearance rates at day 57, with 44% and 54% of patients achieving 100% AK clearance.
- Clinical differences were demonstrated to be statistically significant for all subgroups analyzed, including scalp and face.
- Most TEAEs consisted of application site pruritus or pain that was mild-to-moderate and required no treatment.
- Study participant compliance was excellent, with > 99% completing the full 5-day self-application of ointment.
Key Messages/Clinical Perspectives
- KX2-391 ointment 1% was proven safe and effective for the treatment of adult patients with AK.
- Nearly half (44% and 54% respectively) experience 100% AK clearance with greater improvement measured for the facial area.
- Safety outcomes indicated that most TEAEs were mild-to-moderate and required no treatment.
Presenter disclosure(s): The presenter has reported that he is an investigator for this clinical trial, and that the study is sponsored by Athenex, Inc. The content of the presentation had not been publicly disclosed as of the AAD 2019 presentation, and was provided for research purposes only. Almirall and Athenex are partners in the development program of KX2-391 ointment 1% for actinic keratosis.
Written by: Daniel Bennett, MPH
Reviewed by: Martina Lambertini, MD
CONFERENCE SUMMARIES

Welcome to the Highlights from AAD 2019
Prof. Nellie Konnikov, MD, FAADWe are pleased to present highlights from the 2019 Annual Meeting of the American Academy of Dermatology (AAD). Our meeting was held from March 1 to March 5, 2019 in Washington, DC. The AAD conference … [ Read all ]

 amèrica latina
amèrica latina Canada EN
Canada EN Canada FR
Canada FR Deutschland
Deutschland English
English italiano
italiano português
português