Reports
The Science of Botulinum Toxin Type A: Innovations in Beauty, Health, and Well-being
Presented by: Mark S. Nestor, MD, PhD, FAADVoluntary Professor, Department of Dermatology and Cutaneous Surgery,
Department of Surgery, Division of Plastic Surgery,
University of Miami Miller School of Medicine, Miami, FL, USA
- Knowing the science underlying botulinum toxin type A allows clinicians to compare available therapies and maximize patient outcomes.
- Consideration of “off-label” effects of botulinum toxin type A is important, as clinical effects may impact mood, psychological well-being, and interpersonal relationships.
Botulinum toxin type A injections are the most widely used aesthetic procedure, typically injected to enhance the patient’s facial appearance. Label indications include cosmetic procedures (glabella, frontalis, crow’s feet), spasticity, strabismus, headache, incontinence, and hyperhidrosis. Off-label indications include other cosmetic anatomy, depression, acne and rosacea, pain, and well-being.
Understanding the underlying science is essential to optimizing patient outcomes. Key considerations are efficacy, timing of onset, and duration of effect, all of which hinge upon the agent’s toxin/receptor relationship. Clinicians can also tailor the dosage and distribution of injections sites to improve results. Enhancing the individual patient’s appearance can significantly impact psychological well-being and interpersonal relationships in romantic, social, and professional spheres.
- Clinicians can optimize results for patients treated with botulinum toxin type A by understanding how the underlying science impacts outcomes.
- All botulinum toxin type A treatments have identical underlying science, which facilitates a comparison among commercial options.
- Clinicians can tailor dosage and distribution to achieve maximum benefit in terms of efficacy and duration, while minimizing adverse effects.
- Newly approved prabotulinumtoxin A offers increased duration of efficacy, which is a paramount consideration.
- Botulinum toxin type A treatments can alleviate depression symptoms in patients who did not respond to medication, depending upon facial muscles treated.
- The effect of treatment on mood supports the propositions that facial muscles both express and regulate mood, as well as influence interpersonal interactions.
- For physicians to optimize patient outcomes and compare commercially available treatment options, it is essential to understand the science that underlies botulinum toxin type A [1,2]. All type A toxins act identically, which facilitates this understanding.
- Key patient outcomes are : time to onset, efficacy, and duration of effect. Each of these outcomes is a function of the toxin/receptor relationship and mathematical receptor binding model.
- A consideration of molecular potency is essential to comparing commercially available options. This is not proportional to manufacturer toxin units, and patient specific factors such as age, genetics, and muscle mass impact clinical effect, along with clinician technique.
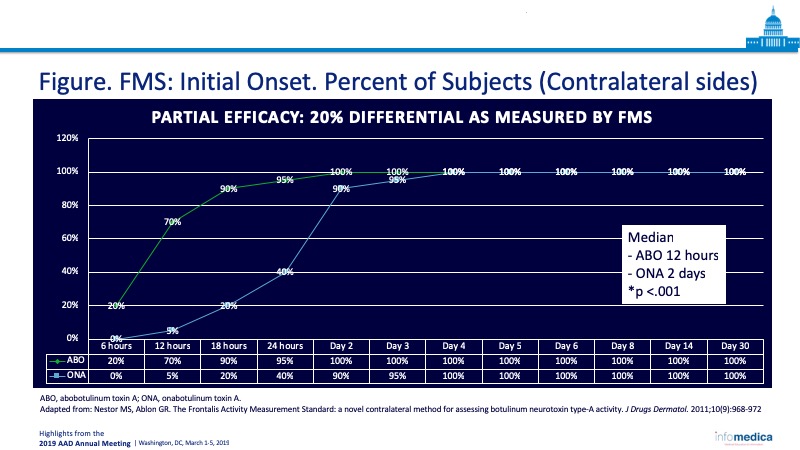
- The objective Frontalis Activity Measurement Standard (FMS) [1] can be used to compare onabotulinum toxin A (ONA) with abobotulinum toxin A (ABO).
- ABO demonstrated greater potency with an earlier onset, and median efficacy of longer duration.
- Adverse events were clinician technique dependent (Figure).
- Patient-important outcomes include balancing a desire for long-lasting results, while achieving a non-frozen appearance.
- Molecular potency can decrease time to onset and increase duration of effect.
- Prabotulinumtoxin A is a recently approved botulinum toxin type A treatment, with greater potency than ONA.
- Clinician technique that allows for a more complete reconstitution can improve distribution, toxin spread, and patient outcomes such as onset, efficacy, and duration of effect. This concept is more useful than that of toxin diffusion.
- Microinjection techniques at a greater number of injection sites also improve toxin spread and distribution and the patient outcomes of onset, efficacy, and duration, as opposed to fewer injection sites.
- Botulinum toxin A can create an appearance perceived as more attractive and approachable and enhance self-esteem. Many studies demonstrate a positive association between physical attractiveness and achievement, health, and well-being [3,4]. In particular, botulinum toxin A injections to the glabella can improve first impressions [5].
- Studies also conclude that treatments that prevent frowning can reduce negative mood [6].
- Treatment with ONA results in a decrease in depression symptoms and improvement in mood for subjects who did not improve on medication for depression symptoms [7]. This supports the conclusion that facial musculature can both express and regulate mood [7]. Due to the impact of social mimicry, the patient with a diminished ability to frown is less likely to receive a social response of frowning and, may therefore, experience a more positive social atmosphere.
- Facial expressions such as the appearance of frowning can be reduced, however, there are potential adverse effects, e.g., crow’s feet, or limited smile expressions.
- For example, the Duchenne smile includes expressions of the orbicularis oculi and zygomatic major muscles, which may be reduced with botulinum toxin A treatment.
- The Duchenne smile is associated with improved mood and social well-being compared to the Pan Am smile, which is limited to the zygomatic major. Treating the glabella only causes improved mood, where treating crow’s feet in addition to glabella does not produce these results. Such treatment also shows a decreased ability to read emotions of others in a manner necessary to empathize [8].
- Sexual satisfaction decreases for women treated with ONA for either glabella, crow’s feet, or both [8].
- A positively perceived facial expression is essential to social, romantic, and professional relationships, with principles of social mimicry creating broader societal implications [8].
Key Messages/Clinical Perspectives
- Advances in botulinum toxin A treatments can improve patient outcomes for this widely used cosmetic dermatology procedure.
- Clinicians can apply scientific knowledge to compare available options and improve clinical techniques to optimize onset, efficacy, and duration metrics.
- Patient outcomes including effects on mood and interpersonal interactions should be considered in selecting among the glabella, crow’s feet, and or other injection sites.
REFERENCES
Presenter disclosure(s): The presenter has reported relationships with the following companies: Aclaris Therapeutics Inc. Actavis Aerolase Allergan, Inc. Almirall Bayer HealthCare Bioderma Biofrontera AG BirchBioMed Brickell Biotech, Inc. Castle Biosciences, Inc. Celgene Corporation Clarisonic Croma-Pharma GmbH Austria Demira DUSA Pharmaceuticals, Inc. Encore Dermatology, Inc. Essence Novel Evolus, Inc. Exeltis Ferndale Laboratories, Inc. Gage Development Company, LLC IntraDerm Pharmaceuticals ISDIN Johnson & Johnson Pharmaceutical Leo Pharma Inc. MC2 Therapeutics Sensus Healthcare Sinclair Pharma SiSaf, Ltd. Sonoma Pharmaceuticals Stratapharma Strathspey Crown Therapeutics Inc. ThermiAesthetics Vanda Pharmaceuticals Inc.
Written by: Daniel Bennett, MPH
Reviewed by: Martina Lambertini, MD
CONFERENCE SUMMARIES

Welcome to the Highlights from AAD 2019
Prof. Nellie Konnikov, MD, FAADWe are pleased to present highlights from the 2019 Annual Meeting of the American Academy of Dermatology (AAD). Our meeting was held from March 1 to March 5, 2019 in Washington, DC. The AAD conference … [ Read all ]

 amèrica latina
amèrica latina Canada EN
Canada EN Canada FR
Canada FR Deutschland
Deutschland italiano
italiano português
português Taiwan
Taiwan