Reports
ALOPECIA AREATA
Oral Janus kinase inhibitors PF-06700841 and PF-06651600 provide clinically evident therapeutic effect at 4 and 6 weeks in patients with alopecia areata and greater efficacy over 24 weeks in patients with a shorter duration of their current alopecia episode: Results of a randomized phase 2a trial
Presented by: Elena Peeva, MD, MSc, FACRCambridge, MA, USA
- Oral Janus kinase (JAK) inhibitors PF-06700841 and PF-06651600 show onset of effect by 6 weeks for alopecia areata (AA) patients.
- Subgroup analyses demonstrate the baseline clinical characteristics specific to duration of AA episode impacts outcomes.
Oral tyrosine kinase 2 (TYK2)/JAK1 inhibitor PF-06700841 and oral JAK3 inhibitor PF-06651600 were studied in this phase 2a clinical trial in moderate to severe AA. Outcome measures specific to timing of onset of effect were compared between the two treatments and placebo. Subgroup analyses of clinical traits specific to AA type and duration were also analyzed.
- To evaluate the onset of action of oral TYK2/JAK1 inhibitor PF-06700841 and oral JAK3 inhibitor PF-06651600 in moderate to severe AA.
- To evaluate the duration of the AA episode and evaluate the relationship between the efficacy of PF-06700841 and PF-06651600 over 24 weeks.
Type of Study
- Double-blind.
- Randomized.
- Multicenter.
- Phase 2a.
- Two ongoing extensions.
Primary Purpose
- The primary purpose of the study was to assess oral TYK2/JAK1 inhibitor PF-06700841 and oral JAK3 inhibitor PF-06651600 in moderate to severe AA.
- The timing of onset of effect and the trait of duration of AA episode were evaluated.
Patient Populations
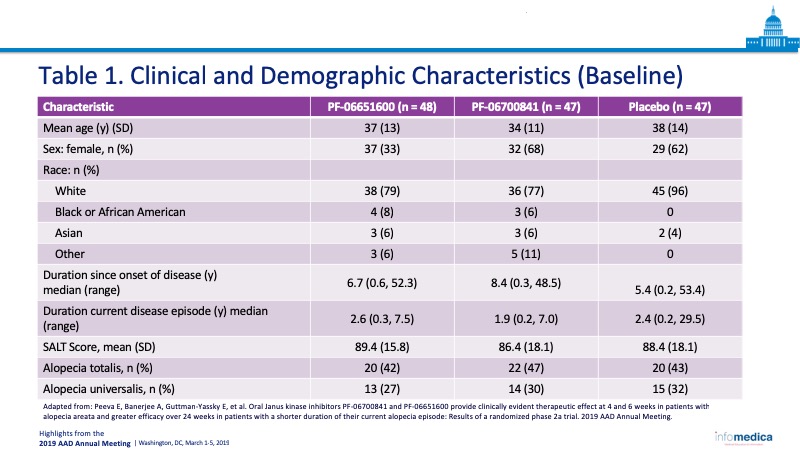
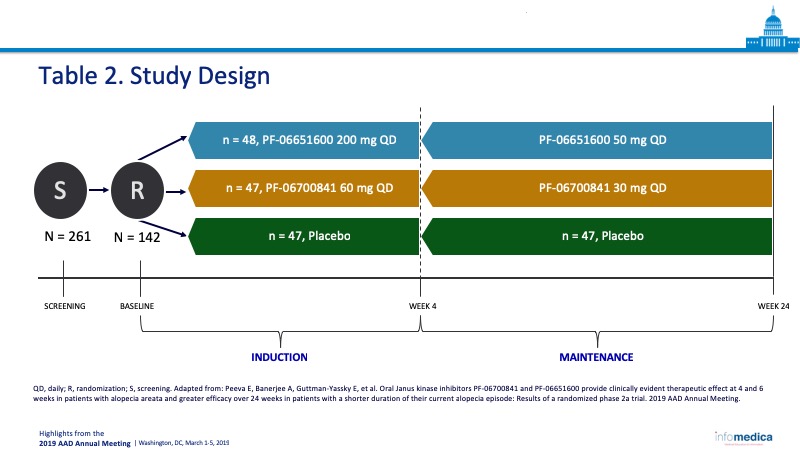
- Total number of enrollees: 142 patients in three cohorts, PF-06700841 (n = 47), PF-06651600 (n = 48), and placebo (n = 47).
- Age: 18-75-year-old patients in three cohorts, with a mean age of 37 years, 34 years, and 38 years respectively.
- Intent-to-treat population.
- Moderate to severe AA (>50% scalp hair loss).
- Includes patients with alopecia totalis and alopecia universalis.
- In the Table 1, the clinical and demographic characteristics. of the patients.
Primary Outcome Measures
- The primary outcome measure was the Severity of Alopecia Tool (SALT) score, with a longitudinal analysis of the change from baseline to identify the first statistically significant difference, comparing PF-06700841, PF-06651600, and placebo.
Secondary Outcome Measures
- The secondary outcome measure was the SALT score subgroup analysis of the change from baseline overtime, comparing PF-06700841, PF-06651600, and placebo to compare the impact of duration of the current AA episode on efficacy.
Drugs/Procedures Used
- Patients were assigned to three cohorts and treated with PF-06700841, PF-06651600, or placebo for the 24 weeks course of the study (Table 2).
- Baseline clinical characteristics were assessed including: duration since onset of disease; duration of current disease episode; SALT score; and whether participant had AA, alopecia totalis, or alopecia universalis.
- SALT score was assessed regularly, with associations between clinical characteristics, disease duration, and treatment cohort measured.
- For the outcome measure of SALT score change, the mean difference from placebo at week 4 was 7.7 for PF-06700841 and 2.9 for PF-06651600.
- The mean difference from placebo at week 6 was 19.6 for PF-06700841 and 12.6 for PF-06651600.
- The subgroup analysis, when stratified by episode duration, indicated that the week 24 SALT score change from baseline, compared to placebo was 53.4 for PF-06700841 and 42.1 for PF-06651600 for participants with a current AA episode <3.5 years.
- The week 24 SALT score change from baseline, compared to placebo was 39.1 for PF-06700841 and 22.7 for PF-06651600 for participants with a current AA episode ≥3.5 years.
- Therefore, the secondary outcome measure assessing the impact of current AA episode duration on efficacy indicated that the response over 24 weeks was greater in participants for whom the current episode of AA was of shorter duration.
- The oral TYK2/JAK1 inhibitor PF-06700841 and oral JAK3 inhibitor PF-06651600 have an onset of effect in moderate to severe alopecia areata at 4 weeks and 6 weeks.
- The response measured at 24 weeks may be greater in participants with baseline measures of current alopecia areata episode that is shorter in duration.
Key Messages/Clinical Perspectives
- The phase 2 trial data for the oral TYK2)/JAK1 inhibitor PF-06700841 and oral JAK3 inhibitor PF-06651600 indicate that they demonstrate efficacy earlier than prior treatments, having an onset of effect in moderate to severe alopecia areata at 4 weeks and 6 weeks respectively.
- Subgroup analyses by clinical characteristics demonstrate that the response may be greater for patients in whom the disease has persisted for shorter duration.
Presenter disclosure(s): The presenter has reported the following disclosure: Pfizer Inc.
Written by: Daniel Bennett, MPH
Reviewed by: Martina Lambertini, MD
CONFERENCE SUMMARIES

Welcome to the Highlights from AAD 2019
Prof. Nellie Konnikov, MD, FAADWe are pleased to present highlights from the 2019 Annual Meeting of the American Academy of Dermatology (AAD). Our meeting was held from March 1 to March 5, 2019 in Washington, DC. The AAD conference … [ Read all ]

 amèrica latina
amèrica latina Canada EN
Canada EN Canada FR
Canada FR Deutschland
Deutschland English
English italiano
italiano português
português